Medical Devices Expected to Change the World by 2025
Search For Schools
When you click on a sponsoring school or program advertised on our site, or fill out a form to request information from a sponsoring school, we may earn a commission. View our advertising disclosure for more details.
New medical innovations emerge every day that improve health and extend lifespans. Much of the credit for such advancements goes to a plethora of new medical device startups and university research labs. There’s ample evidence that this field is only expected to grow; Fortune Business Insights projects revenues for medical device firms will reach $718.92 billion by 2029.
Today, researchers work to create noninvasive therapies, counter treatment-resistant diseases, make medical research safer, and revolutionize organ donation. With so many new devices flooding the market, it can take time to keep track of major game-changers that really move their segment of healthcare forward.
Fret not: this article explores five medical devices reshaping people’s healthcare approach.
Intravascular Lithotripsy (IVL)
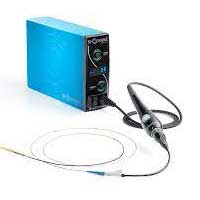
Shockwave Medical has revolutionized calcified plaque removal by repurposing sonic technology used since the 1980s to break up kidney stones. Their intravascular lithotripsy (IVL) device, approved by the FDA, breaks up arterial calcium with sound waves rather than relying on higher-risk approaches such as high-pressure balloons or atherectomy drills that can cause damage to soft tissue and even perforate walls.
How It Works
An IV catheter is inserted in the patient and threaded through to the calcified deposits. A small balloon is inflated to facilitate the energy transfer. Once in place, an electrical discharge from the emitters vaporizes the fluid within the balloon, creating a rapidly expanding & collapsing bubble that generates sonic pressure waves. This cracks calcium deposits, which allows them to flow through the artery and get captured by a stent.
Why It’s a Big Deal
This method of sonically breaking apart hardened deposits within arteries allows for a safer expansion procedure when accompanied downstream with stent placement. Previous procedures run the risk of damaging soft tissue or even puncturing artery walls. IVL therapy, on the other hand, has minimal soft tissue trauma and has been proven effective in over 12,000 patients.
The Details
- Manufacturer: Shockwave Medical
- Cost: Unknown
- Projected Release: Currently available for purchase

In May 2019, Dr. Keith D. Dawkins joined Shockwave as their Chief Medical Officer. He had previously held the post of Global Chief Medical Officer and Executive Vice President at Boston Scientific Corporation from 2012 to 2017.
Over the course of his career, Dr. Dawkins has achieved a multitude of distinctions in clinical practice, research, and academia. His impressive resume includes a Post-Doctoral Research Fellowship at Stanford University as part of the Fulbright Scholarship Program, and he was appointed President of the British Cardiovascular Intervention Society (BCIS). Additionally, his expertise has been sought to serve on regional and national hospital committees, such as with National Institute for Health & Clinical Excellence (NICE). Dr. Dawkins is an unparalleled expert in this field with more than 750 academic publications & presentations referring to various cardiac topics under his name.
CleanSpace HALO

Covid has revolutionized the way healthcare providers and consumers utilize personal protective equipment. In addition to traditional disposable masks, several innovative reusable masks and filter technologies have been developed to protect healthcare professionals, including the CleanSpace HALO. This device has become the gold standard in Personal Respiratory Protection for workers in clinical settings. Since it is worn around the neck, there are no belts, hoses, or waist-mounted battery packs. It can even be sterilized and disinfected for continued use.
How It Works
The CleanSpace HALO is a battery-powered unit that filters the air before delivering it to the breathing zone of a wearer, allowing for clean and purified respiration. The HALO power system is paired with a half mask or hood, depending on the level of protection required. It is a dynamic respirator, thoughtfully designed to adjust its fan speed in tandem with the user’s breathing and to maintain a positive pressure in the mask to protect the user. The available CleanSpace Smart App allows users to check their mask fit anytime. The app also tracks device performance and battery charge.
Why It’s a Big Deal
This device is 40 times more effective at filtration than an N95 mask, giving healthcare unprecedented protection against aerosolized pathogens such as Covid. Not only can it help keep nurses and physicians healthy, it provides peace of mind. It also reduces the waste of single-use masks.
The Details
- Manufacturer: CleanSpace Technology
- Cost: $1,976.70 for the HALO power system and $261.80 for the half-mask
- Projected Release: Currently available for purchase

Mr. Alex Virr joined CleanSpace in 2010 with extensive experience in medical device manufacturing, motor design, flow control systems, and highly regulated quality management systems. Having previously been employed by Telectronics & ResMed, he brought this expertise to help design the CleanSpace HALO. He holds two bachelor’s degrees. The first is in arts from The Australian National University, and the second is in mechanical engineering from the University of New South Wales.
Cala Trio

Patients with essential tremors often face the difficult decision of living with their condition or risking unwanted side effects from pharmacologic treatment options. However, a new approach offered by Cala Health provides an effective and health-conscientious solution for these individuals.
The small FDA-approved wrist-worn device is both medically effective and minimizes physical risk. Clinical trials conducted by Cala Health revealed that 57 percent of participants experienced at least a 50 percent reduction in tremor severity, while 61 percent reported improved ability to carry out basic daily activities such as eating and writing.
How It Works
Utilizing a wrist-worn device, Cala Trio therapy offers an innovative and risk-free solution to essential tremor sufferers. Through electrical stimulation, known as neuromodulation, this revolutionary treatment works to address the source of essential tremors to provide relief without invasive procedures or drugs. It works in about 40 minutes and can provide relief for up to an hour. Patients must receive a prescription for this device as it must be individually calibrated to each patient’s unique brain signal pattern.
Why It’s a Big Deal
Previous essential tremor treatments come with significant pharmacological side effects or complicated surgeries for deep brain stimulation. This new device is non-invasive, with minor side effects such as hand tingling or muscle weakness. With this simple wrist device, patients with essential tremors can regain their independence to perform everyday tasks such as reading, cooking, or writing.
The Details
- Manufacturer: Cala Health
- Cost: Varies based on insurance reimbursement
- Projected Release: Currently available for purchase

During her neuroscientist and engineer training, Cala Health co-founder & chief scientific officer Kate Rosenbluth experienced the need for an efficient treatment option for essential tremor in individuals during her biodesign fellowship at Stanford University.
By collaborating with various neurologists, physicians, and nurses to observe unmet requirements in medicine throughout multiple clinical visits at Stanford Hospital, she was able to identify the possibility of assisting patients affected by hand tremors or essential tremors.
Working with her co-founder Scott Delp, director of the Stanford Neuromuscular Biomechanics Laboratory, she set about reverse engineering movement disorders circuitry outside of the brain. Once they had that figured out, they could develop the Cala Trio to work in those circuitry systems to reduce tremors. She holds a PhD in bioengineering from the University of California, Berkeley.
Bioheart

In November, Bioheart revolutionized personal heart health with a groundbreaking direct-to-consumer EKG device that pairs conveniently with a smartphone.
Available at retailers like Amazon, patients can get unprecedented insight into baseline readings and long-term data for doctor visits or inform their own personal health decisions. The app will also provide personalized insights from long-term data.
How It Works
Bioheart employs cutting-edge cardiac technology and comprehensive analytics to provide a triple view of the heart, enabling personalized insights into the state of one’s cardiovascular health. Patients wear a band around their chest that connects via Bluetooth to an app on their phone. A 48-hour battery life allows for long-term tracking without the need to recharge every day. With no need for prescription medication, this innovative platform uses an individual’s unique data to deliver tailored guidance that is meaningful and effective in caring for their own hearts.
Why It’s a Big Deal
Bioheart brings the convenience of real-time and long-term metrics to both doctors and patients. Previously, long-term data was only available with expensive monitors prescribed by a physician. Now, anyone who wants to keep track of their heart health can purchase one and gain valuable insights that can help improve overall health.
The Details
- Manufacturer: Biotricity
- Cost: $199
- Projected Release: Currently available for purchase

Dr. Waqaas Al-Siddiq, PhD, is the founder, CEO, and chairman of Biotricity. He is an expert in wireless communication and has an extensive background with start-ups. He has a long history of entrepreneurial pursuits and has held multiple design positions in esteemed organizations such as IBM, AMD, and Intel. Furthermore, major platforms like IEEE and National Communication Council conferences have recognized his innovative designs.
His extensive board experience gives him a substantial leadership skill set. He has a master’s in computer engineering from Rochester Institute of Technology and a master’s in business administration from Henley Business School. He also holds a business doctorate specializing in transformative innovations and billion-dollar markets from Henley Business School.
Archived: Five Groundbreaking New Medical Devices in 2020
Aether 1 Bioprinter

The Aether 1 Bioprinter is a 3D printer that lets doctors and researchers create cellular scaffolding for biomedical purposes. Think 3D-printed stents and artificial organs. Unlike most bioprinters, which are designed to serve one primary function, the Aether 1 can print food products, prototypes, and much more in addition to biomedical materials. Modular attachments like microscopes, laser engravers, and hot syringe extruders increase the printer’s versatility all the more while four unique automation features make otherwise time-consuming or complex processes more efficient.
How It Works
Aether 1 prints tissue-like structures using “bioinks,” or gel-like materials compatible with human tissue. Users can print with up to 24 different biomaterials using four different fabrication methods. These unique abilities are spearheading major research in areas like organ replacement, stem cell research, orthology, and wound treatment.
Why It’s a Big Deal
Bioprinting is one of today’s most dynamic medical technology research areas. Researchers and health professionals have thus far printed artificial livers, hearts, bones, and more, but new uses and theories emerge all the time. Harvard University researcher and professor Jennifer Lewis even printed thick tissues containing blood vessels. Some researchers eventually publish open source printing plans and procedures.
Another example of boundary-pushing bioprinting is 4D printing, or the use of biomedical materials that can change that can change shape or behavior in response to certain stimuli. That means one could theoretically print a heart or intestine that contracts. According to the World Economic Forum, such advancements could eventually lead to 3D-printed transplant organs. The devices have already reduced the need for animal testing.
The Details
- Manufacturer: Aether (current status on the company in 2022 is unknown)
- Cost: About $9,000. Aether notes that competitors’ bioprinters usually cost at least $250,000.
- Projected Release: Mid-2017, though Aether already donated dozens of the machines to universities for use in major medical research projects.

Dr. Jennifer A. Lewis is the Hansjörg Wyss Professor of Biologically Inspired Engineering at Harvard University’s John A. Paulson School of Engineering and Applied Sciences and the founder of its Lewis Research Group. She is also a core faculty member at the University’s Wyss Institute for Biologically Inspired Engineering. Dr. Lewis is a materials science expert and major pioneer in engineering cells and tissues using biomaterials. With more than 120 scientific publications and eight patents under her belt, Dr. Lewis recently turned her attention to using multi-nozzle arrays—similar to those found in the Aether 1—to push the boundaries of bioprinting. Among her accomplishments: printing a slab of tissue with blood vessels. Dr. Lewis holds a Doctor of Science in Ceramic Science from MIT.
Kortex VR Device

Fisher Wallace Labs’ Kortex VR is a small brain-stimulation device shown to increase the production of endorphins, mood-regulating serotonin, and sleep-promoting melatonin while reducing that of the stress hormone cortisol. The device attaches to the back strap of any virtual reality headset to create a relaxing, immersive experience that promotes healthy sleep, though its creators note it can be used independently. The Kortex VR relies on the same technology as the Fisher Wallace Stimulator, which is cleared by the FDA to treat depression, anxiety, chronic pain, and insomnia. The device itself, however, was successfully crowdfunded through IndieGoGo (linked above).
How it works
Kortex gently stimulates the brain with patented brainwaves that produce mood- and sleep-promoting neurochemicals. Columbia University psychiatrist Dr. Richard P. Brown told Fisher Wallace that the technology brought relief to more than 70 percent of about 400 severely depressed patients resistant to other therapies. That’s about twice the rate of antidepressants.
Why It’s a Big Deal
The rapidly-growing field of brain stimulation is a often more effective—and less invasive—means of psychiatric treatment compared to most existing therapies. Recent studies suggest it may even improve symptoms of Parkinson’s disease and autism, among other medical conditions. Kortex’s novel VR-integration, portability, accessibility, and comparatively low cost brings brain stimulation technology to a whole new level.
The Details
- Manufacturer: Fisher Wallace Labs
- Cost: $499
- Projected release: Currently available for purchase

Dr. Richard P. Brown is an associate clinical professor of psychiatry and clinical researcher at Columbia University’s College of Physicians and Surgeons, and co-founder of Breath Body Mind health workshops. As one of the first medical professionals to test Kortex, he has a keen professional interest in non-invasive healthcare and serving treatment-resistant patients, particularly those who suffer from depression, anxiety, and post-traumatic stress disorder. He also developed a comprehensive physiological theory dealing with the relationship between yoga breathing and such challenges as insomnia, stress, mood disorders, and difficulties with emotion regulation. Dr. Brown has published more than 100 scientific articles and delivers more than 100 lectures each year globally. He earned his MD from Columbia University and a Certificate of Psychopharmacology from the American Society of Clinical Pharmacology.
TAP Blood Collection Device
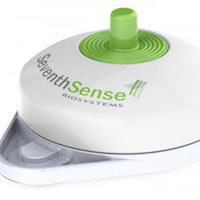
TAP is an FDA-cleared device that collects blood painlessly using an array of tiny needles called microneedles. Users simply push a button and blood is vacuumed into the device; samples are anticoagulated and ready for lab transport. Researchers hope the device can reduce the number of patients who delay or forego medical tests that rely on drawn blood. It could also be a helpful resource for clinical trials, pharmacies, athletic monitoring, and more.
How It Works
According to manufacturer Seventh Sense Biosystems (7SBio), TAP’s spring-loaded ring of microneedles, which are smaller than an eyelash, creates tiny punctures through which blood is drawn. 7SBio CEO Howard Weisman says the needles are deployed and retracted so rapidly that patients do not feel them at all. As for the TAP device, it is about the size of a stethoscope.
Why It’s a Big Deal
Though life-changing for patients terrified of traditional phlebotic needles, it is TAP’s use of microneedles that is especially revolutionary. It is only one of a whirlwind of new and developing devices using them. Researchers are testing a wearable patch called SkinJect, for instance, that uses dissolvable microneedles to treat basal cell and squamous cell skin cancers directly. Basal cell carcinoma accounts for nearly 80 percent of skin cancer diagnoses, or about two million cases a year.
Another forthcoming device called sugarBEAT is a reusable electronic sensor—also applied using a skin patch—that continuously monitors one’s glucose via a non-perceptible current across the skin. The Bluetooth-enabled device lets diabetics access this information anytime with an app. According to University of Bath pharmaceutical sciences professor Dr. Richard Guy, when paired with microneedles, the patches could painlessly inject insulin whenever patients need it, without any action on their parts.
The Details
- Manufacturer: Seventh Sense Biosystems
- Cost: Varies
- Projected release: Second generation, Tap II, is availabe on the market

Dr. Robert S. Langer is the Massachusetts Institute of Technology’s (MIT’s) David H. Koch Institute Professor—the highest honor awarded to faculty members—and co-founder of Seventh Sense Biosystems. With more than 1,000 issued and pending patents licensed or sublicensed to more than 300 medical device, biotechnology, and pharmaceutical companies, MIT calls Dr. Langer the “most cited engineer in history.” He once served on the FDA’s highest advisory board and was profiled by dozens of prominent publications. Dr. Langer received more than 220 major awards. Among them: The United States National Medal of Science and the United States National Medal of Technology and Innovation. Dr. Langer earned a BS in Chemical Engineering from Cornell University and his ScD from MIT.
neuroAD Therapy System

The neuroAD Therapy System could be the world’s first medical device designed to treat mild Alzheimer’s disease. The non-invasive system combines brain stimulation—specifically transcranial magnetic stimulation (TMS)—with mental exercises to improve cognitive performance in patients with the disease. While the neuroAD does not modify Alzheimer’s disease, studies associate treatment with remarkable improvements on the Alzheimer’s Disease Assessment Scale-Cognitive Subscale within 12 weeks. Patients can maintain benefits through occasional, subsequent sessions.
How it works
NeuroAD uses TMS and cognitive exercises to concurrently target areas of the brain affected by Alzheimer’s disease. Transcranial magnetic stimulation uses electromagnetic induction to stimulate small parts of the brain. Electric current is produced by a pulse generator and delivered through a coil placed near the head while patients complete memory-boosting brain exercises. Initial neuroAD treatment consists of 30 hour-long daily treatments followed by occasional maintenance sessions.
Why It’s a Big Deal
Alzheimer’s disease is considered one of medicine’s greatest unmet challenges; the neuroAD Therapy System would be the first medical device to breach those walls. There are only a few drugs available to Alzheimer’s patients, but benefits tend to be limited and accompany painful side effects. As a non-invasive therapy, neuroAD could improve Alzheimer’s symptoms with few side effects. The system can be used independently or in conjunction with drug therapies.
In a press release published on PRWeb, Harvard Medical School professor of neurology Dr. Alvaro Pascual-Leone described neorAD as “the most exciting breakthrough in the field of Alzheimer’s disease research in more than a decade.”
The Details
- Manufacturer: Neuronix Ltd.
- Cost: About $6,000 to $10,000 for a full six-week course of daily treatments.
- Projected Release: As of 2022, the company failed FDA approval and has closed.

Dr. Alvaro Pascual-Leone is a Professor of neurology and director of the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center and Harvard Medical School, where he also serves as program director of the Harvard-Thorndike Clinical Research Center. Dr. Pascual-Leone’s primary research interest: using brain imaging and stimulation to modulate brain plasticity and to document relationships between targeted brain activation and behavior. His work aims to prevent age-related cognitive decline and reduce one’s risk for dementia and neurodevelopmental disorders like Alzheimer’s disease. According to HMS, Dr. Pascual-Leone is ranked no. 1 among authors in the field of TMS and Noninvasive Brain Stimulation. He has several patents and has authored more than 600 scientific papers and several books. Dr. Pascual-Leone earned both his MD and PhD from Albert-Ludwigs University in Freiburg, Germany.
Dr. A.I.
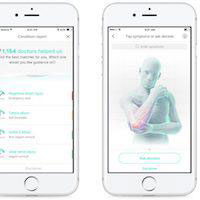
Dr. A.I. connects patients with personalized medical direction through an app downloaded on their smart devices. The platform digitizes healthcare triage, which means it assesses one’s medical risk and recommends the first step toward treatment. Dr. A.I. uses on health parameters like one’s weight, age, and medical history, and a series of follow-up questions to devise those recommendations. While physicians can already use AI-enhanced software to monitor patients, Dr. A.I. is an important step in putting medical AI squarely in patients’ hands. The app aims to improve consumer medical information to minimize misdiagnoses and unnecessary—or potentially dangerous—home treatments.
How It Works
Dr. A.I. uses machine learning, individual patient medical histories, and more than a half-decade of knowledge from 105,000 triage experts to direct patients with medical concerns. Unlike internet search engines often used to research symptoms, Dr. A.I. considers one’s personal medical background to develop highly-targeted questions. It then produces a list of potential causes of one’s symptoms ranked by the seriousness of the conditions. Dr. A.I. also uses a cloud-based digital health platform that connects patients with doctors via phone, text, or video chat. It is available on both Android and iOS devices.
Why It’s a Big Deal
Experts expect artificial intelligence to revolutionize healthcare as we know it. Google began developing AI surgical robots in 2015, for instance, and doctors can already use AI-enhanced software to monitor patients. Dr. A.I. is an excellent example of how accessible such tools are becoming, and how quickly. While the app is a major step in consumer-driven medical triage in the United States, the UK’s National Health Service is already using a similar platform called Babylon to reduce lengthy patient waiting lists by weeding out cases that do not necessarily require a physician visit. Like Dr. A.I., Babylon lets patients monitor their health and ask medical questions using an AI chat tool. Because it is a government-condone app, however, Babylon users can actually order medical kits and tests for at-home diagnostic purposes.
As helpful as it might be, medical use of AI is the subject of ethical debate. Some experts worry devices do not have the personal insight of human healthcare providers and could, therefore, overlook something critical. Nonetheless, AI proponents believe the ease and accessibility of tools like Dr. A.I. can theoretically prompt more patients to mitigate life-threatening medical situations and reduce home-health mishaps and the load of unnecessary doctor visits. The technology is perhaps even more important to rural and economically disadvantaged patients with limited healthcare access.
The Details
- Creator: HealthTap
- Cost: Free, though there may be in-app purchases.
- Projected release: Dr. A.I. made its public debut in early January of 2017. It is currently integrated into their virtual primary and urgent care app.

Ron Gutman is the co-founder and CEO of the Silicon Valley startup HealthTap and an active speaker and curator in the TED community. He is an advisor and angel investor to such companies as Harvard Medical School’s SMArt Initiative and Stanford Medical X. Previously Mr. Gunman served as founder and CEO of the online community of health writers Wellsphere. While a graduate student at Stanford University, Gutman lead a diverse group of faculty and students to research ways to personalize health and increase patient engagement. Several national publications have covered his work in healthcare, innovation, and smiling. Among them: Forbes and the Huffington Post.
The Future of Health Innovation: How to Get Involved
Many of the companies highlighted here are, or were, small startups founded on little more than a very good idea, some investment capital, and sheer will. In other words, the medical device market is no longer reserved for major pharmaceutical companies and labs. The University of Utah has even published a list of instructions for future innovators. Interested, prospective students are encouraged to invest their time in the med-tech professions and degrees that improve one’s chances of getting involved, especially those that deal specifically with biomedical engineering, medical lab technology, and business.


